Acetylene Formula Ethyne is an alternative name to Acetylene. Acetylene is the simplest alkyne as well as is a hydrocarbon. The formula for acetylene is C2H2. The chemical has no saturated state. Two bonds connect the carbon atoms that make up it. Despite its being unstable in its simplest form, it is utilized to solve problems. Acetylene is used extensively in the welding of oxyacetylene, illuminant cutting, soldering and cutting of metals; signaling the precipitation of copper, particularly and acetaldehyde, as well as manufacturing with acetic acids. In this article we will look at how to use the Acetylene Formula, Structure, Hybridization, Properties, Application and much more.
Also Read: a3-b3 formula : algebraic formula
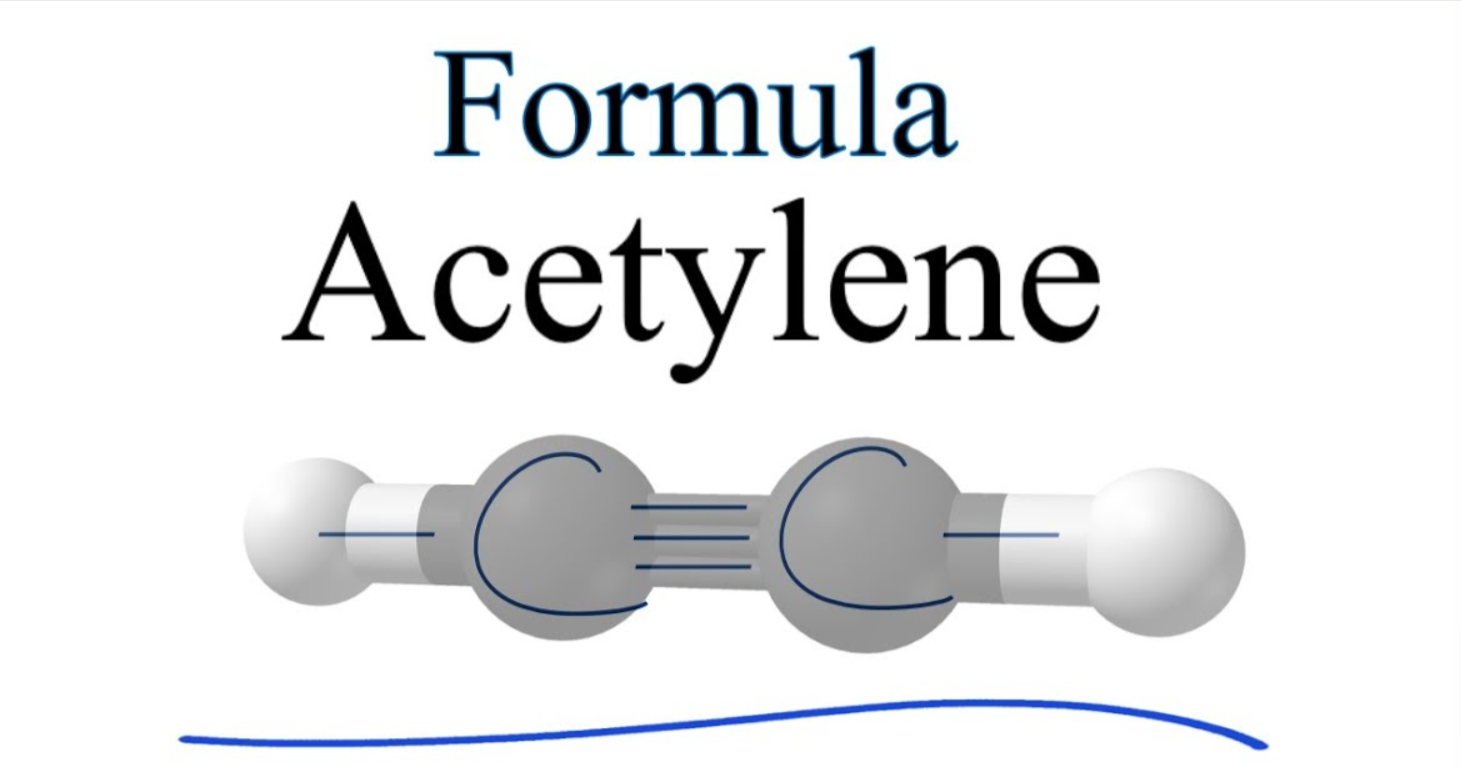
Acetylene Formula
What is Acetylene? Acetylene is also known by the names Narcylen as well as Vinylene comes with an chemical formula of C2H2. Vinylene is a non-colorless gas that has a slight smell that resembles ether. It is easily dissolvable in water, chloroform, acetone and benzene. It is soluble but not completely in alcohol and carbon disulfide. It is much lighter than air and can be used to cause ignition. The long-term exposure to flames or heat can cause containers to crack. If they are exposed to pressures greater fifteen pounds/square inch acetylene pure explodes quickly, regardless of its liquid or solid form.
Acetylene Formula Structure
Acetylene is one of the most fundamental alkyne molecule. It’s a functional group identified by scientists to possess triple bonds.
- The chemical formula of Acetylene is C2H2 Its extended formula is CHCH.
- It is made up from two carbon atoms joined through double bonds.
- In Acetylene, two hydrogen atoms are linked to the carbon atoms that are triple-bonded.
- The molecules of the compound are linear in 180 degrees and contain carbon atoms forming hybrid to form sp. Additionally, both carbons have two orbitals of sp One of which is linked to hydrogen and the other one to the carbon’s basic bond.
- However, it is the case that the triple bond comprised of two bonds which form with no hybridization of the four orbitals of P is orthogonal to linear system.
Acetylene Formula Ethene
Ethene’s molecular formula is C2H4. Ethene is an unsaturated hydrocarbon that has the presence of two carbon molecules and 4 hydrogen atoms joined via two bonds.
- The hydrocarbons that have the formula CnH2n in which n is the carbon atoms’ number are referred to as alkenes.
- The electron dot structure was constructed by keeping in mind the Octet rule that states that throughout the synthesis process of molecules, the atom belonging to a particular element may gain, lose or exchange electrons until it attains the electronic configuration that is stable, with eight electrons within its the valence shell.
- Carbon is home to four valence electrons and hydrogen just one.
- The electron dot structure of Ethene can be shown below.
Acetylene Formula Benzene
This is a ring of aromatics which carbons are linked through double bonds that alternate and hydrogen is connected to carbons.C6H6 is the chemical formula used to describe benzene.
- Benzene contains an average mass of 78.112 grams. Benzene contains hydrogen atoms.
- Since the double bonds that comprise this structure are separated by one bond, it is thought to be a compound of double bonds that are conjugated.
- A circle is an auxiliary symbol inside the hexagon which signifies the six pi electrons.
- Carbon’s six electrons are split into two subshells, namely K as well as L. This instance the K subshell is already full, whereas the L subshell is only containing four electrons. This means it is the L subshell will require four electrons in order to make its Octet.
- Since hydrogen atoms have only one electron, and is situated in the K subshell, and is able to only contain 2 electrons. This means that hydrogen just requires one electron to create its Octet. This means that each carbon atom makes an unidirectional bond with only one hydrogen atom.
Acetylene Formula Hybridization
Triple-bonded groups, such as alkaline compounds and nitriles profit from the hybrid orbital concept. In this article, we will examine what is the structure and composition of acetylene the alkyne with the lowest atomicity.
- The molecule is linear the sense that the four atoms are the same straight line. The carbon-carbon triple bond is the length of 1.20. Both carbons are sp hybridized in an image that is a hybrid of orbital images Acetylene.
- The 2s orbital combines with the 2px orbital within the form of a sp hybridized carbon. This creates two orbitals of sp which can be aligned to an angle of 90 degrees the other (e.g. on an x-axis).
- The orbitals 2py and 2pz remain unhybridized, and are perpendicular to the y as well as the z axes.
- Carbon has one electron that is unpaired in the orbital 2 s and a single pair in the orbital 2 p. Two electrons are unpaired in the s p orbital following hybridization, one pair of electrons in the orbital 2 py and an non-paired electron within the 2 p orbital.
- The orbitals of the s p are parallel to 2 orbitals of p and y. The 2 P Z orbital is perpendicular the plane of the page.
- The C-C sigma bond was formed by the overlap of a single sp orbital from every carbon that has an orbital of 1s from hydrogen. The two C-H sigma bond are created by the overlap between the second orbital that is derived from each carbon, with one orbital that is 1s of hydrogen.
- Every carbon atom continues to have two 2py half-filled and 2pz orbitals, which are perpendicular to one another and also to the sigma bond line. The carbons are joined by the two perpendicular pairs of p orbitals create two pi bonds, which result in the triple bond (one Sigma bond plus 2 pi bonds).
Acetylene Formula Lewis Structure
The central part of the acetylene Lewis structure is made up of both carbon atoms due to the fact that they have a higher value (total 8) than hydrogen atoms (total 2). In the end, each carbon atom is located in the center of the structure and hydrogen atoms are scattered around them.
- The shell that surrounds carbon atoms contains four electrons that are valence. Carbon atoms have two inside the ethyne. Therefore, each Carbon Atom in C2H2 contains eight electrons of valence.
- A shell on the outside of an atom of hydrogen is made up of one the valence electron. This is why Hydrogen Atoms have two electrons called valence.
- Valence electrons are present in C2H2 equals 8+2 = 10 which results in the total of ten electrons valence within the molecular.
The molecules’ octets are completely created in Lewis structure. There aren’t any single electron pairs within the molecular. - Both hydrogen atoms share one of carbon atom’s valence electrons, and create bonds. The duplets of each hydrogen atom are done.
- The octet that is formed by carbon atoms is not complete. To ensure stability, the Carbon atoms form triple bonds in order to share their remaining 3 electrons that are valence.
- Six electrons called valence are used to form the triple bond between carbon atoms. In addition, four electrons are utilized to create an unidirectional link between hydrogen and carbon atoms.
Acetylene Properties
Let’s examine some of the most significant chemical and physical properties of acetylene.
Acetylene Physical Properties
- Acetylene, as pure a form is an odorless and non-colorless gas that has a garlic scent.
- To ship for shipping, we break down any compound using the aid of acetone.
- Acetylene is a chemical with a molar mass of 26.038 grams per mo.
- Acetylene can be found in the temperature of melting as well as boiling at 80.8 degree Celsius, and -84.7 degC, respectively.
- Acetylene containers may explode violently when exposed to flame or heat for a prolonged period of time.
Acetylene is dissolved in acetone water, benzene and chloroform. - Acetylene’s density is 1.097 kg/m3 and is easily measured.
- Carbon disulfide and ethanol both are very solubilized in.
Acetylene Chemical Properties
Because of the electrons that form the triple bond C-C, Acetylene is an extremely active chemical molecule. This is why acetylene has become an effective nucleophile. Here can be described as the properties chemical of acetylene.
- It can go through a vast variety of reactions that produce commercial goods like the acrylic acid, acetylide alcohol and a vinyl compound.
- When it is combined with a metal such as copper, it could also be used in the production of organic compounds.
- Acetylene and sodium react in a chemical reaction that produces Sodium Hydroxyxide, as well hydrogen.
Na + C2H2 – NaHC2 +H2 - If acetylene has been treated with HBr it produces the ethylidene bromide.
CHCH+ HBr – CH2 = CHBr – CH3 CHBr2 (Ethylidene Bromide)
CH3CHCI2 is produced when acetylene reacts with HCI.
C2H2 + HCI – CH2 = CHCI
CH2 = CHCI + HCI – CH3CHCI2
Where Acetylene is located in Nature?
Some bacteria utilize Acetylene as the principal source for acetaldehyde synthesis. The substance is also discovered in natural oil and gas wells together with crude oil as well as other gasses. Additionally it is a part of the atmosphere of our solar planet.
Acetylene Preparation
It is when water reacts with calcium carbonate within the lab to create Acetylene. It was created by the discovery of a reaction in 1862 in the work of Friedrich Wohlerthe.
Method of Acetylene Preparation:
- Within a cylindrical flask put a few pieces from calcium carbide. Making use of the funnel to drop and several droplets of water.
- Acetylene gas is generated and then absorbed by water displacement downwards. The process of hydrolysis in the reaction of calcium carbide is in the following manner:
CaC2+2H2O-Ca(OH)2+C2H2
- The reaction described above takes place at a very high temperature, at around 2000 0C with an electric an arc furnace.
- Impurities like phosphine ammonia, hydrogen sulfide arsine and many more are found in acetylene that is made from calcium carbide.
- All contaminants, except phosphine arsine, are absorbed when passing the gas produced through water.
- Arsine and phosphate are filtered out of gas via transferring the gas through an acidified solution of K2Cr2O7 solution or CuSO4 solution.
- Because acetylene can be almost insoluble with water, the purest gas is gathered through the downward movement of water.
Uses of Acetylene
Let’s take a look at some fascinating uses for Acetylene.
- Acetylene is an important substance that can be transformed into a variety of compounds. Acetylene reacts with water vapor to produce vinyl chloride and acetic acid when it is in contact with the acidic catalyst.
- Due to the high temperature of flames made from acetylene (33000C) Acetylene is widely used in welding processes across a wide range of industries. The flame is utilized for incandescent lighting in a few less developed nations.
- Acetylene is used for the manufacture of rubber. It is used for the vulcanization process of rubber, giving its elasticity and strength. The process involves chemically heating and treating rubber to improve its strength and become more durable.
- Acetylene is used for the production of a range of medicines.
- Acetylene is often utilized in the manufacturing of resins and polymers. Acetylene gas is composed from two carbon atoms. It is highly reactive. When used in the production process, it creates resins and polymers that are extremely durable and durable. This allows them to be used in various applications, such as automobile components, building materials and medical devices.